News – Events
About Us
Algiax Pharmaceuticals is a clinical-stage biotechnology company established in 2011.
It is dedicated to the discovery and development of innovative products to treat diseases with a high unmet medical need.
Algiax’ lead candidate AP-325 is a small-molecule GABAA receptor modulator in clinical development as a therapy for neuropathic pain.
Next to AP-325 Algiax has discovered novel GABAA receptor modulator compounds called Thioacrylamide (ThAc) derivatives. The company is advancing a selection of ThAcs derivatives from its discovery to preclinical characterization.

MANAGEMENT TEAM

Dr. Ingo Lehrke
CEO
Dr. Ingo Lehrke | CEO
Ingo joined the team in early 2019 as CEO with a view to develop and grow the company. He has been a managing director for the past 13 years as well as a shareholder in a drug delivery company. Ingo is both, science and business driven and holds a PhD in pharmaceutics as well as a Master of Business Administration. Having spent over 18 years in the pharmaceutical industry Ingo feels well prepared for this new and exciting task at Algiax Pharmaceuticals.

Dr. Guido Koopmans
CSO
Dr. Guido C. Koopmans | CSO
From the start Guido was responsible for all R&D activities within Algiax, together and in close collaboration with Dr. Birgit Hasse he brought Algiax to the point where it is today. Guido is one of the co-founders of Algiax and is most and for all a scientist with proven technical and managerial experience in the areas of pre-clinical drug discovery and development. He has experience in a GLP, GMP and GCP environment and has highly developed knowledge of most areas of pharmaceutical development from target to Clinical Phase II. He is results driven with an entrepreneurial mindset. Guido is a PhD, with a master degree in Biological Health Science.
Dr. Birgit Hasse
Head of CMS and Regulator
Affairs
Dr. Birgit Hasse | Director CMC and QA
As a co-founder of Algiax, Birgit was from the beginning accountable for all drug substance and drug product activities from preclinical development, manufacturing and formulation development through clinical material supplies for all clinical registration studies. Birgit has 20 years of experience in drug development and profound knowledge of the pharmaceutical and clinical regulatory environment under ICH, GCP, GMP and GLP and is responsible for the quality assurance of all activities across the portfolio/clinical development. Birgit is a PhD, with a master degree in Biology.

Stefan Fischer
Finance and Transaction Advisor
Stefan Fischer | CFO
Stefan Fischer has a degree in Business Administration and more than 15 years of experience in the life science and biotech industries. Stefan was previously the CFO of NewLab BioQuality, the biopharmaceutical analytics company founded by Dr. Schumacher, which was acquired by a large US company.

Advisory Board

Dr. Jürgen Schumacher
Dr. Jürgen Schumacher
Jürgen Schumacher is a renowned entrepreneur. Jürgen co-founded QIAGEN in 1984 and served as member of Qiagen´s management board until 1993. Later he co-founded Evotec, Vivoscience, Algiax Pharmaceuticals as well as other biotech and life science companies. Jürgen has more than 35 years experience in the industry and is a member of numerous biotech associations and is a consultant for the biotechnology activities of North Rhine Westphalia.

Prof. Dr. med.
Claudia Sommer
Prof. Dr. med. Claudia Sommer
Claudia Sommer is a Professor of Neurology at the University of Würzburg, Germany, with a focus on peripheral neurology and pain. She received training in neurology, psychiatry, neuropathology, and experimental anaesthesia. At the University of Würzburg, she serves as a consultant in neurology, organizes outpatient clinics for patients with peripheral neuropathies, headache and pain, and leads the Northern Bavarian Neuromuscular Centre and the Peripheral Nerve Laboratory. Research interests are neuropathic pain, the fibromyalgia syndrome, and the pathophysiology of antibody-mediated diseases. She has written more than 250 original research papers and more than 100 reviews and book-chapters and edited several books. She is active in the development of national and international guidelines on diagnosis and treatment of peripheral neuropathies, and on neuropathic pain. She is a reviewer for scientific journals, the German Research Foundation (DFG) and serves on the editorial boards of several scientific journals. She is a longstanding member of the Peripheral Nerve Society (PNS) and a Board Member of the Inflammatory Neuropathy Consortium (INC), as well as Chair of the Teaching Course Subcommittee of the European Academy of Neurology (EAN). As of Jan 2019, she is President of the German Pain Society and President elect of the International Society for the Study of Pain.

Dr. Klaus-Dieter Langner
Dr. Klaus-Dieter Langner
Klaus-D. Langner holds a Ph.d. in Molecular Biology from University of Cologne. He has more than 30 years of experience in the pharmaceutical industry. Klaus started his career as research scientist at Behringwerke AG in 1986. In 1998 he joined Gruenenthal GmbH, where he worked 10 years as Head of Research. In 2013 Klaus was appointed to the Corporate Executive Board of Gruenenthal GmbH as Chief Scientific Officer where he stayed until 2018.
R & D Overview
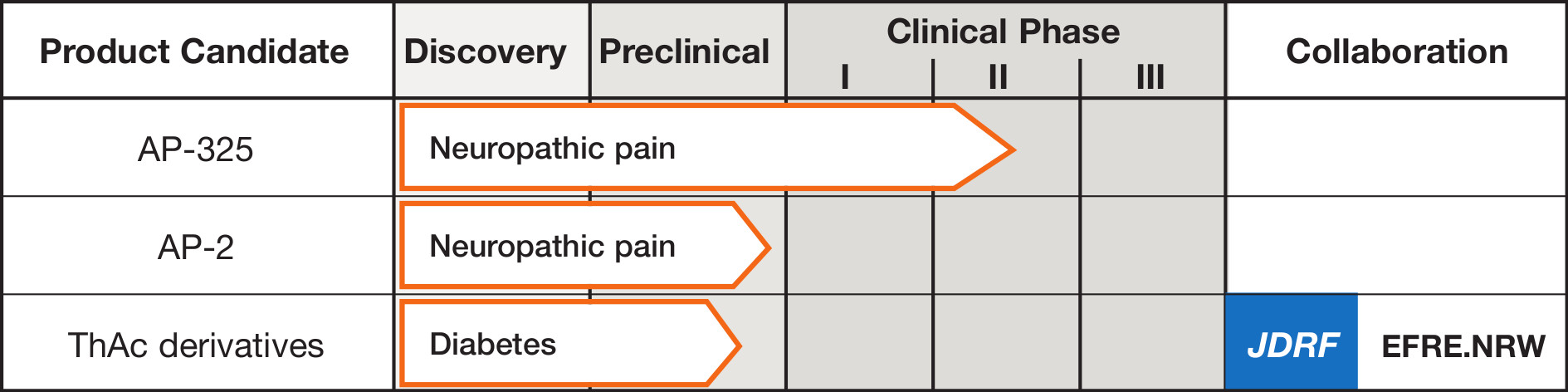
AP-325
Algiax Pharmaceuticals has discovered AP-325, a unique proprietary small molecule drug compound, to treat neuropathic pain. AP-325 acts as a positive allosteric modulator (PAM) of the GABAA-receptor. GABAA receptor PAMs work via allosteric modulation: they do not bind at the same active site as the natural ligand Gamma-aminobutyric acid (GABAA) but affect the receptor by binding at a different site on the protein.
AP-325
Algiax Pharmaceuticals has discovered AP-325, a unique proprietary small molecule drug compound, to treat neuropathic pain. AP-325 acts as a positive allosteric modulator (PAM) of the GABAA-receptor. GABAA receptor PAMs work via allosteric modulation: they do not bind at the same active site as the natural ligand Gamma-aminobutyric acid (GABAA) but affect the receptor by binding at a different site on the protein.
GABAA is a major inhibitory neurotransmitter in the central nervous system. Upon binding, it triggers the GABAA receptor to open its chloride channel to allow chloride ions into the neuron, thus hyperpolarizing the cell and inhibiting the transmission of the nerve action potential, thereby blocking the neuropathic pain inducing pain signal, a mechanism that is strongly enhanced under influence of AP-325. AP-325 is designed to treat a large group of neuropathic pain patients and does not penetrate the brain as such there are no sedative actions that limit their use. Consequently, AP-325 as a non-opioid has a significantly lower potential for addiction or abuse and is truly unique because it is non-sedative, not centrally active and has a long-lasting efficacy.
ThAc
Next to its AP-325 drug molecule, Algiax is investigating Thioacrylamide (ThAc) derivatives in preclinical research. ThAcs bind to the GABAA-receptor at the same binding site as AP-325 and the functional resemblance between ThAcs and AP-325 was confirmed in rodent models. Based on these and other positive results in preclinical research, Algiax is convinced that the ThAcs offer an excellent opportunity as a new treatment approach in diabetes and chronic pain.

ThAc
Next to its AP-325 drug molecule, Algiax is investigating Thioacrylamide (ThAc) derivatives in preclinical research. ThAcs bind to the GABAA-receptor at the same binding site as AP-325 and the functional resemblance between ThAcs and AP-325 was confirmed in rodent models. Based on these and other positive results in preclinical research, Algiax is convinced that the ThAcs offer an excellent opportunity as a new treatment approach in diabetes and chronic pain.
The ThAcs will be further developed in a research grant received from “Der Europäische Fonds für Regionale Entwicklung (EFRE) and North Rhein Westphalia (NRW). The aim of EFRE/NRW Grant is to establish a new center of competence for innovative diabetes therapy (www.komit-nrw.de). The competence center is a sustainable infrastructure of collaborating partners that have the common goal to bring innovative approaches of diabetes therapy rapidly and directly to the patients.


Contact
Adress
Mettmanner Straße 25
Gebäude 9
40699 Erkrath

Mrs. Martina Zmugg
Team Assistant
Phone
Imprint
Information pursuant to § 5 TMG
Algiax Pharmaceuticals GmbH
Mettmanner Straße 25
Gebäude 9
40699 Erkrath
Commercial Register: HRB 23420
Registration Court: Wuppertal
Represented by:
Dr. Ingo Lehrke, Chief Executive Officer
Dr. Guido Koopmans, Chief Scientific Officer
VAT ID:
Sales tax identification number according to § 27a of the Sales Tax Law:
DE815282049
Copyright
Contents and compilations published on these websites by the providers are subject to German copyright laws. Reproduction, editing, distribution as well as the use of any kind outside the scope of the copyright law require a written permission of the author or originator. Downloads and copies of these websites are permitted for private use only.
The commercial use of our contents without permission of the originator is prohibited.
Copyright laws of third parties are respected as long as the contents on these websites do not originate from the provider. Contributions of third parties on this site are indicated as such. However, if you notice any violations of copyright law, please inform us. Such contents will be removed immediately.
Disclaimer
Liability for Contents
As service providers, we are liable for own contents of these websites according to Paragraph 7, Sect. 1 German Telemedia Act (TMG). However, according to Paragraphs 8 to 10 German Telemedia Act (TMG), service providers are not obligated to permanently monitor submitted or stored information or to search for evidences that indicate illegal activities.
Legal obligations to removing information or to blocking the use of information remain unchallenged. In this case, liability is only possible at the time of knowledge about a specific violation of law. Illegal contents will be removed immediately at the time we get knowledge of them.
Liability for Links
Our offer includes links to external third party websites. We have no influence on the contents of those websites, therefore we cannot guarantee for those contents. Providers or administrators of linked websites are always responsible for their own contents.
The linked websites had been checked for possible violations of law at the time of the establishment of the link. Illegal contents were not detected at the time of the linking. A permanent monitoring of the contents of linked websites cannot be imposed without reasonable indications that there has been a violation of law. Illegal links will be removed immediately at the time we get knowledge of them.
Reference

Portraits
On following sites: About us, Team Management and Advisory Board
Copyright: © foto3003.de – Philippi Fotografie

Young fitness woman legs walking. . .
Image number: 202854496
Copyright: © Jo Panuwat D

Young fitness woman legs walking. . .
Image number: 202854496
Copyright: © Jo Panuwat D

Friendly competition. Joyful seniors. . .
Image number: 197318463
Copyright: © Jo Panuwat D


